Cinacalcet Ascend
11 Limitations of Use. Cinacalcet 60 mg is not a controlled substance under the Controlled Substances Act CSA.

These Highlights Do Not Include All The Information Needed To Use Cinacalcet Tablets Safely And Effectively See Full Prescribing Information For Cinacalcet Tablets Cinacalcet Tablets For Oral Use Initial U S Approval 2004
The NDC Code 67877-504-33 is assigned to a package of 10 blister pack in 1 carton 10 tablet film coated in 1 blister pack of Cinacalcet a human prescription drug labeled by Ascend Laboratories Llc.

Cinacalcet ascend. It is supplied by Ascend Laboratories LLC. Risk cannot be ruled out during pregnancy. NDC Number Drug Product Description 67877-0503-30 Cinacalcet 30mg 30 Tabs 67877-0504-30 Cinacalcet 60mg 30 Tabs 67877-0505-30 Cinacalcet 90mg 30 Tabs.
The generic name of Cinacalcet is cinacalcet. NDC Number Status. Triamcinolone Acetonide Cr 01 15gm Tub.
Add to My Lists. Hypercalcemia of malignancy and belongs to the drug class calcimimetics. Ota heti yhteyttä lääkäriin jos olet ottanut enemmän Cinacalcet Orion tabletteja kuin sinun pitäisi.
Provide a proof-of-purchase and we will attempt to match their price. Cinacalcet Orion valmisteen tavanomainen aloitusannos aikuisille on 30 mg yksi tabletti kaksi kertaa vuorokaudessa. Du bestämmer själv vilka kakor som får lagras i din webbläsare genom att kryssa i de kakor som du godkänner nedan.
Cinacalcet 30 mg is not a controlled substance under the Controlled Substances Act CSA. Cinacalcet Ascend Alkem Aurobindo Cipla Mylan Slate Run Piramal Strides Pharma Teva Generic now available Solodyn Medicis Minocycline 55 80 105 mg extended-release tablet Ascend Alkem 80 105 mg Lupin 55 mg Mylan 80 105 mg Teva 80 105. Cinacalcet tablets are not indicated for use in.
Vad Cinacalcet Mylan är och vad det används för. Rx Item-Cinacalcet 90MG 30 Tab by Ascend Pharma USA. The NDC Code 67877-503-01 is assigned to a package of 100 tablet film coated in 1 bottle of Cinacalcet a human prescription drug labeled by Ascend Laboratories Llc.
Rx Item-Cinacalcet 60MG 30 Tab by Ascend Pharma USA. Cinacalcet tablets contain the hydrochloride salt of the active ingredient cinacalcet a positive modulator of the calcium sensing receptor. Hypercalcemia of malignancy and belongs to the drug class calcimimetics.
Cinacalcet is used in the treatment of primary hyperparathyroidism. Drug Name Active Ingredients Strength Dosage FormRoute Marketing Status RLD TE Code Application No. The NDC Code 67877-504-30 is assigned to a package of 30 tablet film coated in 1 bottle of Cinacalcet a human prescription drug labeled by Ascend Laboratories Llc.
The empirical formula for cinacalcet is C 22 H 22 F 3 NHCl with a molecular weight of 3939 gmol hydrochloride salt and 3574 gmol free base. Secondary Hyperparathyroidism HPT in adult patients with chronic kidney disease CKD on dialysis. Cinacalcet is a positive modulator of the calcium sensing receptor indicated for.
The products dosage form is tablet film coated and is administered via oral form. The products dosage form is tablet film coated and is administered via oral form. The NDC Code 67877-505-30 is assigned to a package of 30 tablet film coated in 1 bottle of Cinacalcet a human prescription drug labeled by Ascend Laboratories Llc.
It is supplied by Ascend Laboratories LLC. Sensipar Cinacalcet HCl. Cinacalcet is used in the treatment of primary hyperparathyroidism.
Cinacalcet Mylan innehåller det aktiva innehållsämnet cinakalcet som fungerar genom att kontrollera nivåerna av parathormon PTH kalcium och fosfor i kroppen. 440 rows Cinacalcet is a calcium sensing receptor agonist used to treat secondary. Name Pharmacy Name Email Phone Number.
The National Average Drug Acquisition Cost NADAC wholesale price per unit for NDC 67877-505-30 is. It has one chiral center having an R-absolute configuration. Add to Wish List.
On July 8 2019 Ascend Laboratories LLC Ascend introduced the following drugs into the market. På Fassse använder Lif och våra leverantörer kakor för att säkerställa att webbplatserna fungerar som de ska och för att följa upp och utvärdera användningen av webbplatserna. Risk cannot be ruled out during pregnancy.
CINACALCET HCL 60MG TB 30. The products dosage form is tablet film coated and is administered via oral form. Cinacalcet is a positive modulator of the calcium sensing receptor indicated for.
Add to Wish List. The products dosage form is tablet film coated and is administered via oral form. Rx Item-Cinacalcet 30MG 30 Tab by Ascend Pharma USA.
Jos otat enemmän Cinacalcet Orion valmistetta kuin sinun pitäisi. Add to My Lists. Cinacalcet with NDC 67877-503 is a a human prescription drug product labeled by Ascend Laboratories Llc.
The products dosage form is tablet film coated and is administered via oral form. Secondary Hyperparathyroidism HPT in adult patients with chronic kidney disease CKD on dialysis. Add to Wish List.
Image Not to scale Details. Add to My Lists. The National Average Drug Acquisition Cost NADAC wholesale price per unit for NDC 67877-504-30 is.
Användning av kakor på Fassse. Det används för att behandla problem i det organ som heter bisköldkörteln.

These Highlights Do Not Include All The Information Needed To Use Cinacalcet Tablets Safely And Effectively See Full Prescribing Information For Cinacalcet Tablets Cinacalcet Tablets For Oral Use Initial U S Approval 2004
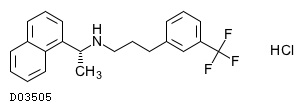
Kegg Drug Cinacalcet Hydrochloride

Pengurangan Harga 1 September 2020 Berita 2021

Ndc 67877 454 Itraconazole Itraconazole

Cinacalcet Ascend 60 Mg Filmtabletten 84 St Docmorris
What Is Cinacalcet Tablet Used For

Pdf Study Design And Baseline Characteristics Of Patients On Dialysis In The Ascend D Trial

Buy Cinacalcet Cinacalcet 30 Mg 1 Ascend Laboratories Llc

These Highlights Do Not Include All The Information Needed To Use Cinacalcet Tablets Safely And Effectively See Full Prescribing Information For Cinacalcet Tablets Cinacalcet Tablets For Oral Use Initial U S Approval 2004

These Highlights Do Not Include All The Information Needed To Use Cinacalcet Tablets Safely And Effectively See Full Prescribing Information For Cinacalcet Tablets Cinacalcet Tablets For Oral Use Initial U S Approval 2004

Pdf Baseline Characteristics Of Subjects Enrolled In The Evaluation Of Cinacalcet Hcl Therapy To Lower Cardiovascular Events Evolve Trial

These Highlights Do Not Include All The Information Needed To Use Cinacalcet Tablets Safely And Effectively See Full Prescribing Information For Cinacalcet Tablets Cinacalcet Tablets For Oral Use Initial U S Approval

Cinacalcet Ascend 30 Mg 28 St Shop Apotheke Com



